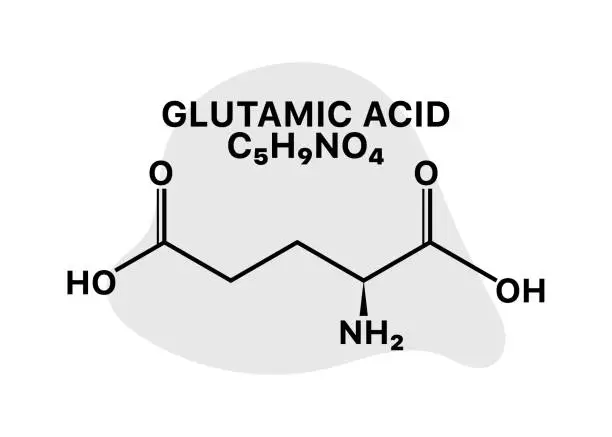
L-Glutamic Acid, a pivotal amino corrosive in our bodies, assumes a critical part in different biochemical cycles. As we dig into the universe of amino acids and their changes, one inquiry frequently emerges: Could glutamic corrosive be phosphorylated? This article will investigate the entrancing domain of glutamic corrosive phosphorylation, its suggestions, and the more extensive setting of amino corrosive adjustments in natural frameworks.
The Chemistry of L-Glutamic Acid
Amino acid glutamic acid, normally known as glutamic corrosive, is named a trivial amino corrosive that assumes a urgent part in a wide cluster of metabolic pathways. Its sub-atomic construction includes a carboxylic corrosive side chain, which separates it from the other 19 standard amino acids. This one-of-a-kind trademark improves its flexibility in different biochemical responses, permitting it to partake in fundamental changes, for example, phosphorylation, which is urgent for controlling protein capability and action.
In its free structure, glutamic corrosive fills in as a vital synapse inside the focal sensory system. It is imperative for working with signal transmission between neurons, going about as a significant excitatory synapse that advances the correspondence of electrical motivations. Besides, glutamic corrosive is an antecedent to gamma-aminobutyric corrosive (GABA), one more basic synapse known for its inhibitory capabilities. This transaction between glutamic corrosive and GABA is fundamental for keeping an equilibrium in mind action, impacting temperament, discernment, and by and large emotional well-being.
Past its jobs in neurotransmission, glutamic corrosive is likewise engaged with protein union and the digestion of carbs and fats. It is pivotal for the amalgamation of different biomolecules, including nucleotides and amino acids, which are essential for cell development and fixing. The complex idea of glutamic corrosive features its importance in keeping up with ideal physical processes and supporting different cell processes, eventually adding to by and large well-being and prosperity. All things considered, it is a subject of progressing research, especially in regions connected with cerebrum well-being, neurodegenerative sicknesses, and metabolic problems.

Phosphorylation: A Key Biochemical Modification
Phosphorylation, characterized as the expansion of a phosphate gathering to a particle, is a broadly perceived and vital post-translational change that happens all through natural frameworks. Because it has the potential to significantly alter the properties of proteins, this modification plays a significant role in regulating various cellular processes. Phosphorylation can alter a protein's function, localization within the cell, and interactions with other molecules by adding a phosphate group. This affects signaling pathways, enzyme activity, and cellular dynamics as a whole.
L glutamic acid powder typically takes place within proteins on particular amino acid residues, with serine, threonine, and tyrosine being the most common targets. These amino acids have hydroxyl (-OH) groups, which make them ideal locations for phosphate groups to be attached by kinase enzymes. The subsequent changes can enact or deactivate proteins, permitting cells to answer quickly to different boosts and keep up with homeostasis.
It is interesting to note that, even though serine, threonine, and tyrosine are the primary amino acids that are associated with phosphorylation, recent studies have shown that other amino acids, such as glutamic acid, may also be subject to phosphorylation in certain situations. This brings up a charming issue: Could glutamic corrosive be phosphorylated? The potential for glutamic corrosive to go through phosphorylation could have critical ramifications for how we might interpret its part in cell flagging and metabolic guidelines. Glutamic acid may have an impact on a wide range of physiological functions and contribute to the intricate network of signaling pathways if it is indeed phosphorylated. In this manner, investigating the phosphorylation of glutamic corrosive might uncover new experiences into its natural importance and extend our cognizance of protein alterations in cell guideline.
Glutamic Acid Phosphorylation: Possibilities and Implications
The phosphorylation of glutamic corrosive is for sure conceivable, yet more uncommon than the phosphorylation of serine, threonine, or tyrosine. A phosphodiester bond between the phosphate group and the carboxylic acid side chain of glutamic acid is created during this process, which is known as glutamyl phosphorylation.
L-glutamic acid has been seen in different organic settings, including bacterial cell wall amalgamation and certain enzymatic responses. For example, a few microorganisms use glutamyl phosphate as a transitional in the biosynthesis of proline, an amino corrosive pivotal for protein structure and osmotic equilibrium.
In eukaryotic cells, glutamyl phosphorylation assumes a part in the guideline of specific catalysts. For instance, the catalyst glutamine synthetase, which catalyzes the transformation of glutamate to glutamine, can be directed through phosphorylation of its glutamic corrosive buildups. This adjustment can influence the protein's action and steadiness, showing the expected meaning of glutamic corrosive phosphorylation in cell processes.

The ramifications of glutamic corrosive phosphorylation stretch out past individual proteins. This alteration can impact protein cooperations, cell flagging pathways, and metabolic cycles. As exploration in this space advances, we might uncover new jobs for glutamyl phosphorylation in both typical cell capability and illness states.
All in all, while glutamic corrosive phosphorylation is more uncommon than the phosphorylation of other amino acids, it addresses a significant and entrancing area of biochemical examination. The capacity of glutamic corrosive to go through phosphorylation adds one more layer to its as of now flexible job in natural frameworks, featuring the intricacy and tastefulness of cell processes.
As we keep on investigating the complexities of amino corrosive adjustments, including the phosphorylation of L-glutamic corrosive, we open up new roads for understanding and possibly controlling cell capabilities. In fields like biotechnology, where companies like HSF Biotech are at the forefront of innovation, this knowledge could have far-reaching effects.
Conclusion
L-Glutamic Acid, an essential amino corrosive in our bodies, can for sure go through phosphorylation, albeit this change is more uncommon than the phosphorylation of serine, threonine, or tyrosine. Glutamyl phosphorylation assumes parts in bacterial cell wall combination, enzymatic responses, and the guideline of specific proteins. This cycle adds one more aspect to the all-around pivotal job of glutamic corrosive in natural frameworks, highlighting its significance in cell organic chemistry.

L-Glutamic corrosive is a white glasslike strong or white powder with a marginally sharp taste. It's dissolvable in water and insoluble in ethanol and ether. In its unadulterated structure, it has a sub-atomic recipe of C5H9NO4 and an atomic load of 147.13 g/mol. It's broadly utilized in the food business as a flavor enhancer and in drug and restorative ventures. For additional data about L-Glutamic corrosive and its applications, if it's not too much trouble, get in touch with us at aaron@healthfulbio.com.
References
1.Meister, A. (1974). Glutamine synthetase of mammals. The enzymes, 10, 699-754.
2.Czonka, L. N., & Hanson, A. D. (1991). Prokaryotic osmoregulation: genetics and physiology. Annual review of microbiology, 45(1), 569-606.
3. Hunter, T. (2000). Signaling—2000 and beyond. Cell, 100(1), 113-127.
4.Besant, P. G., & Attwood, P. V. (2005). Mammalian histidine kinases. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1754(1-2), 281-290.
5.Deutscher, J., Francke, C., & Postma, P. W. (2006). How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiology and Molecular Biology Reviews, 70(4), 939-1031.
Send a Message
You May Like
0